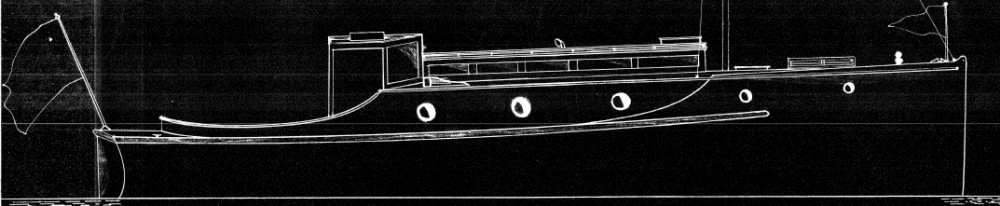
BLUE SEAS
The above photo popped up in my ‘odds and sods’ file – it is dated late Jan 2022 so may have been from when I was helping deliver – Korawai to the BOI. But the photos code tells me it was taken on a Samsung device and I do not touch those, so maybe one of the other crew.
Anyway the launch is most likely named – Blue Seas, can anyone tell us if that is her name and any more intel about her.
INPUT ex Graham Hunter – She is still named BLUE SEAS Waiheke Island is also on the transom. She is now on a Mooring in Whakatakata Bay By OBC
25-07-2022 Input below from Chris Cotter (co-owner)
MY LIPS ARE SEALED – NO NAMES
If you need more proof on why not to bond your wooden boat and attach anodes – have a look below – luckily it was very localised. Very scary, the rudder shaft and surrounding area was VERY close to just not being there and you can guess what would have happened.
And if you need more read the most referenced story on the WW site – link below
https://waitematawoodys.com/2017/12/30/electro-chemical-damage-in-wooden-boats-update-revisited/







28-07-2022 UPDATE – Looking a lot healthier 🙂


30-07-2022 UPDATE – All fixed 🙂